Light passing through a colloidal dispersion such as smoky or foggy air will be reflected by the larger particles and the light beam will be visible.
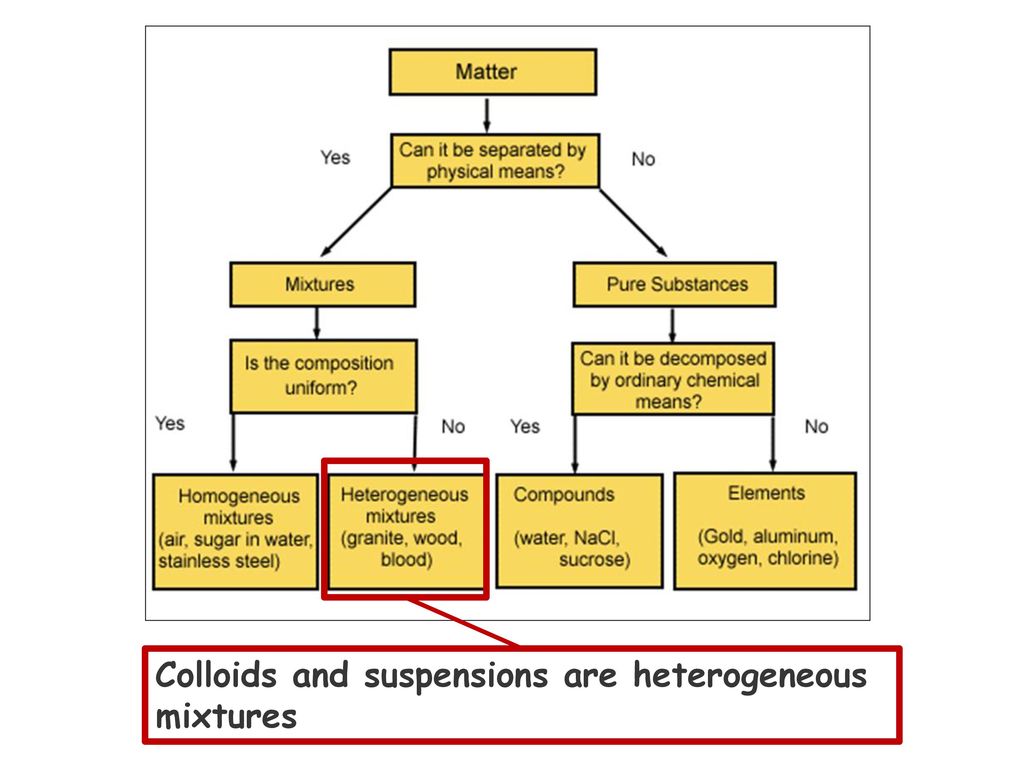
Is granite a solution suspension or colloid.
The dissolving agent is the solvent.
The two parts in every colloid mixture are its particles and the dispersing medium and the particles are spread evenly in in the medium which can also be solid liquid or gas.
A mixture is an association of several substances.
The colloidal solution is the intermediate between true solution and suspension though it is also in the liquid phase.
A colloid is a type of solution in which the particle size of the solute is bigger than that of a true solution but smaller than that a suspension.
A colloid suspension is a suspension that has microscopic particles suspended in another substance.
An example of a solution is saltwater.
A pond isn t a element compound solution colloid or heterogeneous mixture.
Milk blood soap starch solution ink jelly.
Suspensions solutions and colloids are two examples of such mixtures.
A solution is a homogeneous mixture of two or more components.
Is this a.
The particles are spread evenly throughout the dispersion medium which can be a solid liquid or gas.
A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension.
Colloids are homogenous mixtures where the particles are small enough that they stay suspended.
Solutions suspensions colloids and other dispersions are similar but have characteristics that set each one apart from the others.
Colloidal solutions are heterogeneous.
The particles in a colloid land in size between those in a solution and a suspension and may be solid liquid or gas.
The key difference between suspension and colloid is that the particles in a suspension are larger than the particles in a colloid.
So it s either a suspension or a homogenous mixture my vote is suspension.
How do you remove adhesive stains that have come through to the top of your granite.
The heterogeneous mixture of two or more substances where the size of the particles lies between 1 1000 nm is known as a colloidal solution.
A colloid is intermediate between a solution and a suspension.
A solution is a homogenous mixture of two or more substances where one substance has dissolved the other.
While a suspension will separate out a colloid will not.
Since the components in a mixture do not chemically bind together we can physically separate them by filtration precipitation evaporation.
Sand in water is an example of a suspension.
Definition of colloidal solution.